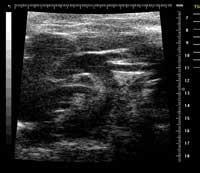
Ephoran Multi Imaging Solutions Preclinical Services is a contract research organization (CRO) located in the Bio Industry Park of Canavese at Colleretto Giacosa (Torino, Italy), offering pre-clinical imaging services. Nonclinical tests to evaluate the safety of new compounds mandatory include animal experiments. Both ethical and economical considerations are now driving towards reduction of the animal use. A contribution to good science and RRR may come from nonclinical imaging. Various imaging modalities have been applied both in toxicological and pharmacological evaluations in normal and pathological animal models. Advances in imaging techniques allow the same animal to be monitored repeatedly over time, thereby reducing animal numbers seeing what is happening during and after treatment by using less invasive experiments. In addition, with the reduction of the number of experiments and the number of animals involved it is possible to measure and to compare the changes that happen to a single animal over time, instead of needing to study separate animals at different points through the process. This reduces the number of experiments needed to carry out and the number of animals involved. As an example of our experience in this field, Rat foetuses from teratology studies preserved in Bouin's fluid and adult males with induced bowel disease were examined by a 7T MRI Bruker system. The cardiovascular system of ApoE mice was explored by MRI and US (Visualsonics Vevo 770) to detect atherosclerotic plaques. 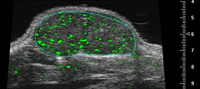 Non-destructive examination of teratological samples can be performed in approx. 20 min per set of foetuses with high definition of organ details. Rat's colon wall has been clearly depicted by MRI and pathology-induced wall thickening was enhanced with the use of a contrast medium. Mouse carotid arteries were well defined by both MRI and US with sufficient details to identify atherosclerotic lesions. Non-invasive techniques, namely Magnetic Resonance Imaging and Echography could be used for toxicological studies. The assessment of changes in the same animal would allow intra- (not inter-) individual comparison, similarly to the clinics. In addition, contrast media are available for nonclinical studies, which can depict not only the anatomical details, but also organ functions. Nonclinical imaging is thus proposed as a contribution both to reduction and refinement in the animal use for safety assessment.
Non-destructive examination of teratological samples can be performed in approx. 20 min per set of foetuses with high definition of organ details. Rat's colon wall has been clearly depicted by MRI and pathology-induced wall thickening was enhanced with the use of a contrast medium. Mouse carotid arteries were well defined by both MRI and US with sufficient details to identify atherosclerotic lesions. Non-invasive techniques, namely Magnetic Resonance Imaging and Echography could be used for toxicological studies. The assessment of changes in the same animal would allow intra- (not inter-) individual comparison, similarly to the clinics. In addition, contrast media are available for nonclinical studies, which can depict not only the anatomical details, but also organ functions. Nonclinical imaging is thus proposed as a contribution both to reduction and refinement in the animal use for safety assessment.
